Electro-reduction of nitrogen on molybdenum nitride: structure, energetics, and vibrational spectra from DFT
Abstract
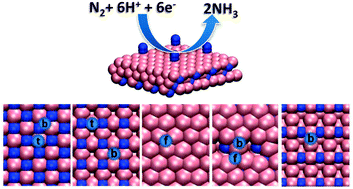
We used density functional theory to study the electrochemical conversion of nitrogen to ammonia on the (001), (100/010), (101), and (111) surfaces of γ-Mo2N. Based on the calculated free energy profiles for the reduction of nitrogen by the associative and dissociative mechanisms, reactivity was found to decrease in the order (111) > (101) > (100/010) ≈ (001). Namely, the cell potentials needed to drive the reduction to ammonia increase in the following order: −0.7 V on (111), −1.2 V on (101), and −1.4 V on (100/010) and (001) surfaces. The (111) surface was found to be the most reactive for nitrogen due to (i) its ability to adsorb the N2 in the side-on position which activates N–N bonding and (ii) its high affinity for N-adatoms which also prevents accumulation of H-adatoms on the catalytic surface at low cell potentials. We have also calculated vibrational frequencies of different NxHy species adsorbed on various γ-Mo2N surfaces. The frequencies are found to depend strongly on the type of the binding sites available on the crystal facets. A comparison of the calculated frequencies with the frequencies of the corresponding species in transition metal complexes and other metal surfaces shows that the frequencies of several signature modes fall in a similar region and might be used to assign the spectra of hydrogen and nitrogen containing surface species on different metal surfaces.

 Please wait while we load your content...
Please wait while we load your content...