Structural, electronic, and photophysical properties of thieno-expanded tricyclic purine analogs: a theoretical study†
Abstract
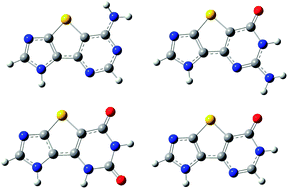
Modified forms of DNA are under intense research because of their potential applications in nanotechnology and medical science. In the present work, comprehensive theoretical investigations into the structural, electronic, and optical properties of four newly designed thieno-expanded base analogs, namely ttA, ttG, ttX, and ttHX, have been performed. The results are compared against the findings obtained for the natural ones. Geometrically, ttA and ttG have nonplanar ground-state geometries caused by the pyramidalization of the amino groups, while ttX and ttHX have planar geometries. Electronically, the ionization potentials and HOMO–LUMO gaps are smaller than natural ones, while the electron affinities are larger than natural ones. The nature of the low-lying excited states is discussed, and it was found that the lowest transitions are of ππ* nature and were mainly dominated by the configuration HOMO → LUMO. The calculated excitation maxima are 283, 302, 294, and 290 nm for ttA, ttG, ttX, and ttHX, respectively, and they are greatly red-shifted compared with natural bases. In the gas phase, the fluorescence from them would be expected to occur around 291, 331, 317, and 323 nm, respectively. The effects of micro-hydration, bulk water solution, and base pairing with their complementary natural bases on the low-lying electronic transitions of these modified bases were also examined.

 Please wait while we load your content...
Please wait while we load your content...