Exploring multi-dimensional coordinate-dependent diffusion dynamics on the energy landscape of protein conformation change
Abstract
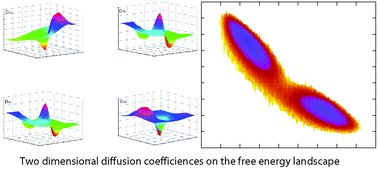
We explore the multi-dimensional diffusion dynamics of protein conformational change. We found in general that the diffusion is anisotropic and inhomogeneous. The directional and positional dependence of diffusion have significant impacts on the protein conformational kinetics: the dominant kinetic path of conformational change is shifted from the naively expected steepest decent gradient paths. The kinetic transition state is shifted away from the transition state. The effective kinetic free energy barrier height, determining the kinetic rate of the conformational change, is shifted away from the one estimated from the thermodynamic free energy barrier. The shift of the transition state in position and value will modify the phi value analysis for identification of hot residues and interactions responsible for conformational dynamics. Ongoing and future experiments can test the predictions of the model.
- This article is part of the themed collection: The free energy landscape: from folding to cellular function

 Please wait while we load your content...
Please wait while we load your content...