Solved? The reductive radiation chemistry of alanine†
Abstract
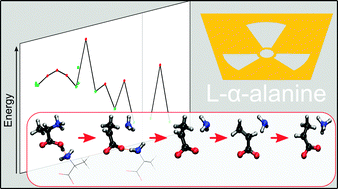
The structural changes throughout the entire reductive radiation-induced pathway of L-α-alanine are solved on an atomistic level with the aid of periodic DFT and nudged elastic band (NEB) simulations. This yields unprecedented information on the conformational changes taking place, including the protonation state of the carboxyl group in the “unstable” and “stable” alanine radicals and the internal transformation converting these two radical variants at temperatures above 220 K. The structures of all stable radicals were verified by calculating EPR properties and comparing those with experimental data. The variation of the energy throughout the full radiochemical process provides crucial insight into the reason why these structural changes and rearrangements occur. Starting from electron capture, the excess electron quickly localizes on the carbon of a carboxyl group, which pyramidalizes and receives a proton from the amino group of a neighboring alanine molecule, forming a first stable radical species (up to 150 K). In the temperature interval 150–220 K, this radical deaminates and deprotonates at the carboxyl group, the detached amino group undergoes inversion and its methyl group sustains an internal rotation. This yields the so-called “unstable alanine radical”. Above 220 K, triggered by the attachment of an additional proton on the detached amino group, the radical then undergoes an internal rotation in the reverse direction, giving rise to the “stable alanine radical”, which is the final stage in the reductive radiation-induced decay of alanine.

 Please wait while we load your content...
Please wait while we load your content...