Dominance of the triplet mechanism in the electron spin polarisation of slowly reacting triplets
Abstract
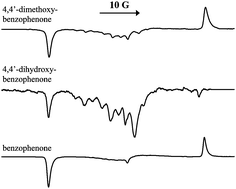
For the triplet mechanism (TM) of electron spin polarisation in photochemically generated free radicals to dominate, the chemical reaction has to be very fast and capable of competing with the electron spin-lattice relaxation process of the triplet. This is a well-established condition. Here we report a counter example of electron spin polarisation, where this condition appears to be violated, and a dominant spin polarisation characteristic of the TM is observed, even when the triplet reacts very slowly. The system studied is the photoreduction of 4,4′-dihydroxy-benzophenone (DHBP) in 2-propanol producing the DHBP ketyl radical and the (CH3)2C˙OH radical. At room temperature, electron spin polarisation of the time-resolved EPR (TREPR) spectra of this system is dominated by the radical pair mechanism involving ST0 mixing, as the rate of the hydrogen abstraction reaction cannot compete with the spin-lattice relaxation of triplet DHBP. However, with the lowering of temperature, TREPR spectra dominated by a net emissive polarisation are seen, even though the rate of the reaction remains slow. Existence of this single-phase, hyperfine-line independent emissive polarisation due to the TM is established by the simulation of their TREPR spectra at −48 °C. To explain the increased contribution of the TM with the lowering of temperature, we proposed that the presence of two hydroxyl groups of DHBP could form hydrogen bonds with the solvent and steadily increase the micro-viscosity experienced by DHBP appreciably as the temperature was lowered. This would progressively increase the rotational correlation time of triplet DHBP, enabling the TM route to contribute increasingly to the radical generation. TREPR experiments with 4,4′-dimethoxy-benzophenone in 2-propanol, where similar hydrogen bonding is not possible, showed the spectra to be dominated by the ST0-RPM with little contribution of a net emissive signal. This observation qualitatively confirmed our proposal of enhanced micro-viscosity experienced by DHBP in 2-propanol at low temperatures, resulting in the enhanced emissive spin polarisation due to the TM, even though the chemical reaction remains slow.

 Please wait while we load your content...
Please wait while we load your content...