Phase evolution of magnetron sputtered nanostructured ATO on grid during lithiation–delithiation processes as model electrodes for Li-ion battery
Abstract
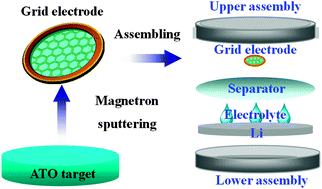
Sb-doped SnO2 (ATO) nanostructured thin films were deposited on holey carbon grids by magnetron sputtering at room temperature. Li/electrolyte/ATO cells were assembled by using the deposited ATO grids as test electrodes. The phase component of the ATO electrodes deposited on grids before and after induction at different charge–discharge stages was characterized by using a transmission electron microscope. The results of the investigation show that the nanostructured ATO thin films undergo a reversible lithiation–delithiation process: the decomposition of SnO2 and the occurrence of metallic Sn followed by the formation of an Li–Sn alloy during the discharge process, and then the reversible de-alloying reaction of the Li–Sn alloy and Sn reaction with Li2O, and even partial formation of SnO2 during charge process. The work also shows that the method deposited the active materials directly on the holey carbon grids is a simple and effective way for the investigation of the phase evolution of the electrodes in electrochemical cells.

 Please wait while we load your content...
Please wait while we load your content...