Between a reactant rock and a solvent hard place – molecular corrals guide aromatic substitutions†
Abstract
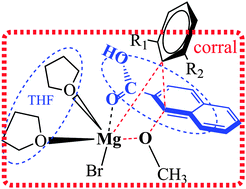
A novel reaction mechanism is presented for an ortho-magnesium carboxylate driven aromatic nucleophilic substitution in naphthoic acids, supported by high-level density functional theory. Results show that the rate-determining aspects involve an R-group transfer from a Grignard reagent Mg-atom to the C1-atom on a naphthalene ring. This transfer is moderated by a molecular corral comprised of two solvent THF molecules and the naphthoic acid, which collectively marshal the R-group into position. The CAM-B3LYP method was employed together with the all-electron DZVP basis set. Solvent was treated using an implicit dielectric continuum (PCM method) and IDSCRF atomic-radii. Further evolved solvent models were also investigated, consisting of explicit solvating particles forming a primary solvation layer framing the reaction center. Reaction barriers obtained are in close agreement with experimental trends, with R-group substituent-identity tempering repulsion with the molecular corral, in-turn modulating the free-energy barriers. Partitioning of the dynamic bases of entropy contribution to free-energy was central to the successful experimental–theoretical synergy.

 Please wait while we load your content...
Please wait while we load your content...