The interaction of beryllium with benzene and graphene: a comparative investigation based on DFT, MP2, CCSD(T), CAS-SCF and CAS-PT2
Abstract
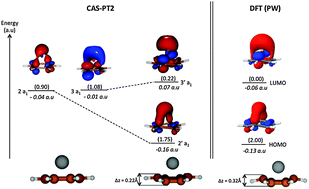
The interaction of beryllium with benzene, graphene and graphitic compounds involves multi-reference electronic states, Jahn–Teller distortion, charge transfer and van der Waals interactions. This is investigated herein using periodic and molecular models at different levels of theory: (i) the second-order Møller–Plesset (MP2) perturbation theory, (ii) the coupled cluster method with inclusion of single double and perturbative triple excitations (CCSD(T)), (iii) the complete active space self-consistent field (CAS-SCF) and (iv) the complete active space with perturbation theory truncated at the 2nd order (CAS-PT2). Molecular and periodic Density Functional Theory (DFT) methods are also used. The two major failures of DFT are addressed with regard to the beryllium benzene and graphene interaction: the degeneracy problem is the source of no specific problem while the delocalization error causes DFT with the Perdew Burke, Ernzerhof functional plus the Grimme correction (DFT/PBE-D2) to be over-binding by about 0.4 eV at a short-range. The agreement between DFT/PBE-D2 and wave-function based methods is nevertheless good; DFT/PBE-D2 provides an accurate description of the electronic structure of the system. By the end of this work, we shall get a better insight into the mechanisms leading beryllium to physisorb on graphene and to chemisorb into the bilayer of graphite.

 Please wait while we load your content...
Please wait while we load your content...