Thermal and energetic processing of ammonia and carbon dioxide bearing solid mixtures
Abstract
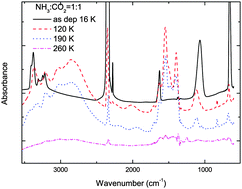
We present new experimental results on thermal and ion irradiation processing of frozen ammonia–carbon dioxide mixtures. Some mixtures were deposited at low temperatures (T ≈ 16 K). Upon warming up to 160 K, complex chemical reactions occur leading to the formation of new molecules and, in particular, of ammonium carbamate. We also show that the same species are produced when water is the dominant species in the ternary mixture with ammonia and carbon dioxide. The samples have been irradiated with 144 keV S9+ ions at 16 K and 50 K. Also in this case, new chemical species are formed as e.g. ammonium formate, CO and OCN−. The results are discussed in the light of their relevance to the chemical evolution of ices in the interstellar medium and in the solar system. In particular, we suggest searching for them among the gas phase species sublimating from grains around young stellar objects and from the cometary nuclei approaching the Sun.
- This article is part of the themed collection: Astrochemistry

 Please wait while we load your content...
Please wait while we load your content...