A new generation of cysteine derivatives with three active antioxidant centers: improving reactivity and stability†
Abstract
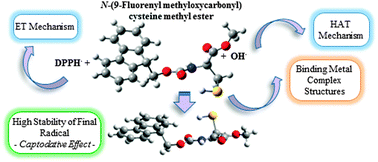
The development of new antioxidants with enhanced activity constitutes a very active research field as it can contribute to the improvement of human health. Although the antioxidant activity occurs through different mechanisms, usually most of the antioxidant molecules present a unique active center which is able to react following a specific way. To overcome this weakness and in the belief that the coupling of different antioxidant groups is a good strategy to obtain multipotent antioxidants, the effect of introducing different N-protective groups on the cysteine core is evaluated by using DFT. As a result, in this work we present a multicenter antioxidant, N-(9-fluorenylmethyloxycarbonyl)cysteine methyl ester 8, able to fight efficiently through different mechanisms against free radicals independently of their nature. This antioxidant appears to be the first one of a promising new class of multipotent antioxidants with three operative centers: Cα that is a good hydrogen donor, the Fmoc group that is a good electron donor and the all-around thiol group. Besides, its neutral radical shows a high stability due to the captodative effect in such a way that the subsequent toxic effects would be avoided. Then, its experimental radical-trapping antioxidant activity postulates compound 8 as a prototype of antioxidants more versatile and efficient than N-acetylcysteine, ascorbic acid or Trolox.

 Please wait while we load your content...
Please wait while we load your content...