Conformational propensities and dynamics of a βγ-crystallin, an intrinsically disordered protein†
Abstract
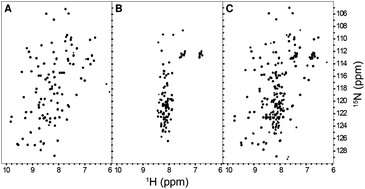
The three-dimensional folded structure of a protein has been considered essential for its function. However, recently many proteins have been identified to function without having a definite structure and they have been classified as intrinsically disordered proteins (IDPs). Recently, we have identified a βγ-crystallin domain in the genome of a marine bacterium called Hahella chejuensis on the basis of known sequence signatures. This protein, called Hahellin, was characterized by NMR spectroscopy as an IDP, which upon Ca2+-binding was shown to undergo a large conformational transformation and acquires a typical βγ-crystallin fold. In this paper, we have characterized this IDP by a combined use of NMR and Replica Exchange Molecular Dynamics simulation and found it to be in a highly dynamic, inter-converting population having a molten globular state with the C-terminal Greek key motif relatively more flexible as compared to its N-terminal counterpart. Network analysis and clustering on the observed conformational ensemble showed a heterogeneous mixture of eleven distinct clusters, classified into near-native and far-native populations, which are not in equilibrium. Several conformational clusters display an increased propensity for helical conformation(s) and a decreased β-strand propensity, which is consistent with the NMR observations made on this protein. The negatively charged Ca2+-coordinating residues form parts of the highly flexible polypeptide stretches, and thus act as seeds for the origin of different conformational clusters observed. This study thus helps us to understand the relationship between the role of conformational dynamics and the structural propensities of the intrinsically disordered state of apo-Hahellin.

 Please wait while we load your content...
Please wait while we load your content...