Novel chiral ionic liquids: physicochemical properties and investigation of the internal rotameric behaviour in the neat system†‡
Abstract
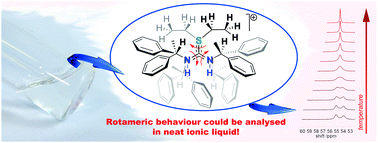
Optically active S-alkyl-N,N′-bis((S)-1-phenylethyl)thiouronium salts, abbreviated as (S)-[Cnpetu]Y (where Y is an anion; n = 1, 2, 3, 4, 6, 8, 10, 12 or 16), have been prepared and studied by a broad spectrum of analyses. This consists of density, viscosity, and conductivity determination, followed by a discussion of relevant correlations. Unusual trends depending on the S-alkyl chain length were documented for (S)-[Cnpetu][NTf2] series (where [NTf2]− = bis{(trifluoromethyl)sulfonyl}amide), including the viscosity decreasing with increasing chain length, and the conductivity showing a maximum between the S-butyl and the S-hexyl derivative. In addition, a hindered rotamerism of the thiouronium cation in dmso-d6 solution was recognised by 1H and 13C NMR techniques. Thorough analysis of NMR spectra confirmed that the main contribution comes from rotation about the partial double C–S bond. For the first time, a neat thiouronium ionic liquid system has been subjected to quantitative analysis of hindered rotamerism by dynamic NMR coalescence studies, with estimated activation energy for rotation of 63.9 ± 0.4 kJ mol−1. Finally, the application of (S)-[Cnpetu]Y salts as chiral discriminating agents for carboxylates by 1H NMR spectroscopy was further investigated, demonstrating the influence of the S-alkyl chain length on chiral recognition; (S)-[C2petu][NTf2] ionic liquid with the mandelate anion gave the best results.

 Please wait while we load your content...
Please wait while we load your content...