Ionic liquid modulation of swelling and LCST behavior of N-isopropylacrylamide polymer gels†
Abstract
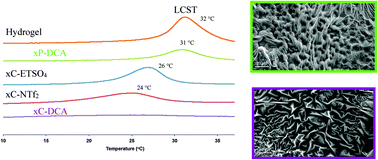
The physicochemical properties of free-standing cross-linked poly(N-isopropylacrylamide) (pNIPAAM) gels, generated in the presence of the Ionic liquids (ILs), 1-ethyl-3-methylimidazolium [C2mIm]+ salts of ethylsulfate [EtSO4]−, dicyanamide [DCA]−, bis(trifluoromethylsulfonyl)imide [NTf2]−, and trihexyltetradecylphosphonium dicyanamide ([P6,6,6,14][DCA]) are described. The Lower Critical Solution Temperature (LCST) of the resulting ionogel was found to vary between 24–31 °C. The behaviour of swelling is found to be as high as 31.55% (±0.47, n = 3) from the initial dehydrated state, while 28.04% (±0.42, n = 3) shrinking from the hydrated swollen state is observed. For ionogels based on the [DCA]− anion an unexpected complete loss of the shrinking behaviour occurs, attributed to water interactions with the nitrile group of the [DCA]− anion. Scanning Electron Microscopy also reveals distinct morphological changes, for example [C2mIm][EtSO4] displays a highly porous, nodule type morphology, efficiently pre-disposed for water uptake.

 Please wait while we load your content...
Please wait while we load your content...