(Pb,Cd)–O covalency in PbTiO3–CdTiO3 with enhanced negative thermal expansion
Abstract
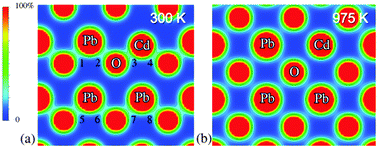
Recently experiments have found that negative thermal expansion is a common phenomenon in PbTiO3-based materials, and their negative thermal expansion is affected by various substitutions. Interestingly, Cd substitution in PbTiO3 has a unique effect in enhancing negative thermal expansion compared with any other A-site substitutions. Therefore, studying Cd substitution in PbTiO3, the role of which still remains unclear, would bring us deeper understanding on the nature of the negative thermal expansion of PbTiO3-based materials. Structure calculations, density of states, Bader analysis and the minimum electron density of Pb1−xCdxTiO3 supercells have been reported on the chemical bond through first-principles calculations here. We found that the hybridization between (Pb,Cd)–O orbitals exists in tetragonal phase. Furthermore, the hybridization between Cd–O orbitals is stronger than that between Pb–O orbitals, and Cd–O covalency promotes the average A-site hybridization. Simultaneously, the average bulk coefficient of thermal expansion is negative and inversely proportional to the Cd substitution amount. So, (Pb,Cd)–O covalency in the tetragonal Pb1−xCdxTiO3 is responsible for the nature of enhanced negative thermal expansion in accordance with our previous experimental investigations.

 Please wait while we load your content...
Please wait while we load your content...