Vaporisation and thermal decomposition of dialkylimidazolium halide ion ionic liquids†
Abstract
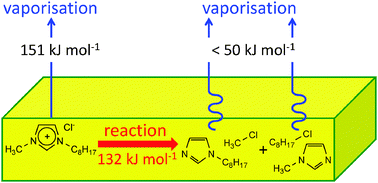
Vaporisation and liquid phase thermal decomposition, TD, of two halide ion ionic liquids, 1-octyl-3-methylimidazolium chloride, [C8C1Im]Cl, and 1-octyl-3-methylimidazolium iodide, [C8C1Im]I, are investigated using temperature programmed desorption (TPD) line of sight mass spectrometry (LOSMS) at ultra-high vacuum (UHV). The ability to use MS to distinguish between vaporisation and TD allows the thermodynamics/kinetics of both vaporisation and TD to be investigated within the same experiments. Vaporisation of both halide ion ionic liquids is demonstrated. For both [C8C1Im]Cl and [C8C1Im]I the vapour is shown to be composed of neutral ion pairs (NIPs). The enthalpy of vaporisation at temperature T, ΔvapHT, was experimentally determined as ΔvapH455 = 151 ± 10 kJ mol−1 for [C8C1Im]Cl and ΔvapH480 = 149 ± 8 kJ mol−1 for [C8C1Im]I. Extrapolation of ΔvapHT to the reference temperature, 298 K, gave ΔvapH298 = 166 ± 10 kJ mol−1 for [C8C1Im]Cl and ΔvapH298 = 167 ± 8 kJ mol−1 for [C8C1Im]I, higher than most ΔvapH298 values measured to date for other [C8C1Im]+-containing ionic liquids. In addition, predictions of ΔvapH298 for other halide ion ionic liquids are made. Liquid phase TD is shown to proceed via nucleophilic substitution to give two sets of products: 1-octylimidazole and methylhalide, and 1-methylimidazole and 1-octylhalide. The activation energy of TD at a temperature T, Ea,TD,T, is measured for the nucleophilic substitution of [C8C1Im]I to give methyliodide; Ea,TD,480 = 136 ± 15 kJ mol−1. Ea,TD,T is measured for the nucleophilic substitution of [C8C1Im]Cl to give methylchloride; Ea,TD,455 = 132 ± 10 kJ mol−1. The fact that ΔvapHT and Ea,TD,T are the same (within error) for both ionic liquids is commented upon, and conclusions are drawn as to the thermal stability of these ionic liquids.
- This article is part of the themed collection: 5th Congress on Ionic Liquids

 Please wait while we load your content...
Please wait while we load your content...