Ground state spectroscopy of hydroxyquinolines: evidence for the formation of protonated species in water-rich dioxane–water mixtures†
Abstract
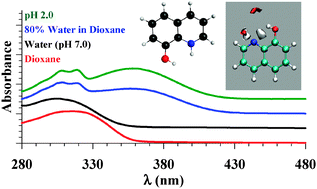
We have recently used 6-, 7-, and 8-hydroxyquinolines (HQs) as fluorescent probes to study the binding mechanism in one of the drug binding sites of human serum albumin. In the present work we study the absorption spectra of the HQ molecules in neat and binary mixtures of dioxane and water in order to identify the different tautomeric species in the ground state. This study should help in identifying the environment in nanocavities of macromolecules when HQs are used as local reporters. The enol form is shown to be the only tautomer for the three HQs in dioxane and water, with the exception of 7HQ in which both the enol and the zwitterion tautomers exist in equilibrium in water. The results are confirmed by the density functional theory (DFT) calculations using the B3LYP method with a 6-311++G(2d,p) basis set. In water-rich dioxane mixtures, all HQs are protonated. The results were confirmed by comparing the absorption spectra in binary solvents with those in acidic and basic aqueous solutions, and by DFT calculations of the Franck–Condon S1 ← S0 transitions. The number of water molecules solvating the polar sites in each HQ molecule was estimated from the spectral change in the binary solvent mixtures, and structures were calculated by DFT. Mapping the water density around the polar sites in each HQ using molecular dynamics (MD) simulations shows well-defined hydrogen bonds around the N-heteroatom in each HQ molecule. Water density is only well-defined around the hydroxyl group in 8HQ. The MD simulations indicate free rotation of the OH group in 6HQ and 7HQ, and the stability of the cis-isomer in 8HQ. The results point to the unique spectral signatures of 7HQ in water which make this molecule a potential probe to detect the presence of water in nanocavities of macromolecules, and to the ability of the three HQs to detect acidic media in binding sites.

 Please wait while we load your content...
Please wait while we load your content...