Light intensity dependence of the kinetics of the photocatalytic oxidation of nitrogen(ii) oxide at the surface of TiO2†
Abstract
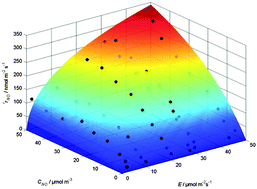
Air pollution by nitrogen oxides represents a serious environmental problem in urban areas where numerous sources of these pollutants are concentrated. One approach to reduce the concentration of these air pollutants is their light-induced oxidation in the presence of molecular oxygen and a photocatalytically active building material which uses titanium dioxide as the photocatalyst. Herein, results of an investigation concerning the influence of the photon flux and the pollutant concentration on the rate of the photocatalytic oxidation of nitrogen(II) oxide in the presence of molecular oxygen and UV(A) irradiated titanium dioxide powder are presented. A Langmuir–Hinshelwood-type rate law for the photocatalytic NO oxidation inside the photoreactor comprising four kinetic parameters is derived being suitable to describe the influence of the pollutant concentration and the photon flux on the rate of the photocatalytic oxidation of nitrogen(II) oxide.

 Please wait while we load your content...
Please wait while we load your content...