A chemical reaction controlled mechanochemical route to construction of CuO nanoribbons for high performance lithium-ion batteries†
Abstract
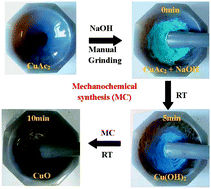
We reported a chemical reaction controlled mechanochemical route to synthesize mass CuO nanosheets by manual grinding in a mortar and pestle, which does not require any solvent, complex apparatus and techniques. The activation of chemical reactions by milling reactants was thus proved, and the energy from mechanical grinding promotes the fast formation of CuO nanoribbons. The resultant materials have preferential nanoscale ribbon-like morphology that can show large capacity and high cycle performance as lithium-ion battery anodes. After 50 cycles, the discharge capacity of CuO nanoribbon electrodes is 614.0 mA h g−1, with 93% retention of the reversible capacity. The thermodynamic reactions of the CuO battery showed size-dependent characterization. The microstructures of CuO nanosheets and reaction routes can be controlled by the ratio of NaOH/CuAc2 according to the chemical reactions involved. The intact nanoribbon structure, thin-layer, and hierarchical structures endow present CuO materials with high reversible capacity and excellent cycling performances. The simple, economical, and environmentally friendly mechanochemical route is of great interest in modern synthetic chemistry.

 Please wait while we load your content...
Please wait while we load your content...