Ribbon aromaticity in double-chain planar BnH22− and Li2BnH2 nanoribbon clusters up to n = 22: lithiated boron dihydride analogues of polyenes†
Abstract
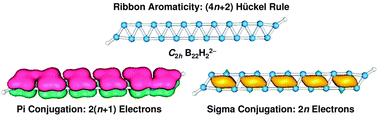
We report an extensive density-functional theory and coupled-cluster CCSD(T) study on boron dihydride dianion clusters BnH22− (n = 6–22) and their dilithiated Li2BnH20/− salt complexes. Double-chain (DC) planar nanoribbon structures are confirmed as the global minima for the BnH22− (n = 6–22) clusters. Charging proves to be an effective mechanism to stabilize and extend the DC planar nanostructures, capable of producing elongated boron nanoribbons with variable lengths between 4.3–17.0 Å. For the dilithiated salts, the DC planar nanoribbons are lowest in energy up to Li2B14H2 and represent true minima for all Li2BnH20/− (n = 6–22) species. These boron nanostructures may be viewed as molecular zippers, in which two atomically-thin molecular wires are zipped together via delocalized bonds. Bonding analysis reveals the nature of π plus σ double conjugation in the lithiated DC nanoribbon Li2BnH20/− (n up to 22) model clusters, which exhibit a 4n pattern in adiabatic detachment energies, ionization potentials, and second-order differences in total energies. Band structure analysis of the infinite DC boron nanoribbon structure also reveals that both π and σ electrons participate in electric conduction, much different from the monolayer boron α-sheet in which only π electrons act as carriers. A concept of “ribbon aromaticity” is proposed for this quasi-one-dimensional system, where regular π versus σ alternation of the delocalized electron clouds along the nanoribbons results in enhanced stability for a series of “magic” nanoribbon clusters. The total number of delocalized π and σ electrons for ribbon aromaticity collectively conforms to the (4n + 2) Hückel rule. Ribbon aromaticity appears to be a general concept in other nanoribbon systems as well.

 Please wait while we load your content...
Please wait while we load your content...