Electrooxidation of methanol in an alkaline fuel cell: determination of the nature of the initial adsorbate†
Abstract
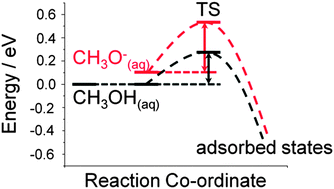
It is essential to correctly determine the nature of the initial adsorbate in order to calculate the pathway for any given reaction. Recent literature provides conflicting information on the first step in the methanol decomposition pathway. This work sets out to establish what role the solution and the surface have to play in the initial adsorption–deprotonation process. Density functional theory (DFT) calculations, in combination with a cluster-continuum model approach are used to resolve the nature of the adsorbing species. We show that methanol is the dominant species in solution over methoxide, and also has a smaller barrier to adsorption. The nature of the surface species is revealed to be a methanol–OH complex.

 Please wait while we load your content...
Please wait while we load your content...