Three-dimensional ordered macroporous MnO2/carbon nanocomposites as high-performance electrodes for asymmetric supercapacitors
Abstract
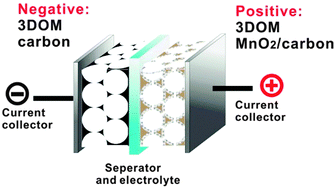
MnO2/carbon composites with ultrathin MnO2 nanofibers (diameter of 5–10 nm) uniformly deposited on three dimensional ordered macroporous (3DOM) carbon frameworks were fabricated via a self-limiting redox process. The MnO2 nanofibers provide a large surface area for charge storage, whereas the 3DOM carbon serves as a desirable supporting material providing rapid ion and electron transport through the composite electrodes. Cyclic voltammetry, galvanostatic charge–discharge and electrochemical impedance spectroscopy (EIS) were used to characterize the capacitive performance of these composites. Optimization of the composition results in a composite with 57 wt% MnO2 content, which gives both a high specific capacitance (234 F g−1 at a discharge current of 0.1 A g−1) and good rate capability (52% retention of the capacitance at 5 A g−1). An asymmetric supercapacitor was fabricated by assembling the optimized MnO2/carbon composite as the positive electrode and 3DOM carbon as the negative electrode. The asymmetric supercapacitor exhibits superior electrochemical performances, which can be reversibly charged and discharged at a maximum cell voltage of 2.0 V in 1.0 M Na2SO4 aqueous electrolyte, delivering both high energy density (30.2 W h kg−1) and power density (14.5 kW kg−1). Additionally, the asymmetric supercapacitor exhibits an excellent cycle life, with 95% capacitance retained after 1000 cycles.

 Please wait while we load your content...
Please wait while we load your content...