An efficient buffer-mediated control between free radical substitution and proton-coupled electron transfer: dehalogenation of iodoethane by the α-hydroxyethyl radical in aqueous solution†
Abstract
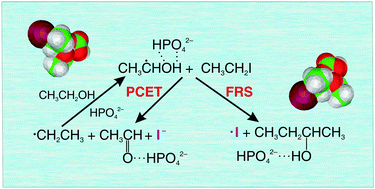
A remarkable buffer-mediated control between free-radical substitution (FRS) and proton-coupled electron transfer (PCET) is demonstrated for the reaction between iodoethane and the α-hydroxyethyl radical in neutral aqueous solution in the presence of bicarbonate or phosphate buffer. The reaction is initiated by the γ-radiolysis of the water solvent, and the products, either the iodine atom (FRS) or anion (PCET), are analysed using ion chromatographic and spectrophotometric techniques. A detailed insight into the mechanism is gained by employing density functional theory (M06-2X), Møller–Plesset perturbation treatment to the second order (MP2), and multireference methods (CASSCF/CASPT2). Addition of a basic buffer anion is indispensable for the reaction to occur and the competition between the two channels depends subtly on its proton accepting affinity, with FRS being the dominant channel in the phosphate and PCET in the bicarbonate containing solutions. Unlike the former, the latter channel sustains a chain-like process which significantly enhances the dehalogenation. The present systems furnish an example of the novel PCET/FRS dichotomy, as well as insights into possibilities of its efficient control.

 Please wait while we load your content...
Please wait while we load your content...