Enhanced electrochemical performance of MWNT@MnO2 composites in polymerized ionic liquids†
Abstract
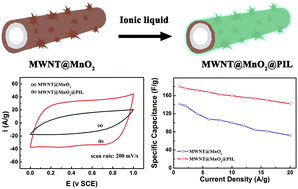
In this article, we report a newly devised composite electrode for electrochemical capacitors, by depositing manganese dioxide (MnO2) on the surface of carbon nanotubes via a spontaneous redox reaction. X-ray diffraction and transmission electron microscopic studies demonstrated a homogeneous coating of layered MnO2 on the surface of the carbon nanotubes. The MWNT@MnO2 composite electrode displayed enhanced capacity performance and rate capability as compared to the MnO2 electrode. Introduction of a thin layer of polymerized ionic liquid on the surface of the composite electrode enhanced the utilization rate of MnO2 and improved the conductivity of the resultant electrode. Electrochemical tests confirmed the augmented capacity performance and rate capabilities of the polyion-coated composite electrode.

 Please wait while we load your content...
Please wait while we load your content...