Electrochemical ammonia production on molybdenum nitride nanoclusters†
Abstract
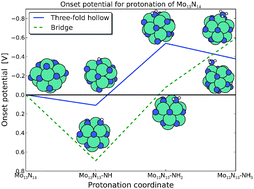
Theoretical investigations of electrochemical production of ammonia at ambient temperature and pressure on nitrogen covered molybdenum nanoparticles are presented. Density functional theory calculations are used in combination with the computational hydrogen electrode approach to calculate the free energy profile for electrochemical protonation of N2 and N adatoms on cuboctahedral Mo13 nanoparticles. Pathways for electrochemical ammonia production via direct protonation of N adatoms and N2 admolecules with an onset potential as low as −0.5 V and generally lower than −0.8 V on both a nitrogen covered or a clean Mo nanoparticle. Calculations presented here show that nitrogen dissociation at either nitrogen vacancies on a nitrogen covered molybdenum particle or at a clean molybdenum particle is unlikely to occur under ambient conditions due to very high activation barriers of 1.8 eV. The calculations suggest that the nitrogen will be favored at the surface compared to hydrogen even at potentials of −0.8 V and the Faradaic losses due to HER should be low.

 Please wait while we load your content...
Please wait while we load your content...