Solvatochromism of pyranine-derived photoacids†
Abstract
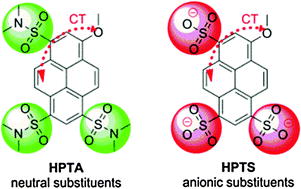
Photoacidity is frequently found in aromatic alcohols where the equilibrium dissociation constant increases by some orders of magnitude upon electronic excitation. In this study we investigated the solvatochromism of a family of recently synthesized super-photoacids and their methylated counterparts based on pyrene. The chemical similarity of these molecules on the one hand and their differing photoacidity with pKa* values between −0.8 and −3.9 on the other allow for gaining insights into the mechanisms contributing to excited-state proton transfer. Three different solvent scales, namely Lippert–Mataga, Kamlet–Taft and Catalán, were independently employed in this study and gave consistent results. We found the strongest correlation of the excited-state acidity with the dipolarity of the excited state, pem ranging from −1775 cm−1 to −2500 cm−1, and a concomitant change in the permanent dipole moment of roughly 14 Debye. Spectral changes due to varying basicity of the solvent, which probes the conjugated property of the solute, are found to be less indicative of the graduation of excited-state acidity, i.e. bem values between −700 and −1200 cm−1. The solvent acidity is the only parameter with a distinct influence on the electronic spectra of the deprotonated species. The low values of aem ∼ 400 cm−1, which are 3–4× smaller than aabs and aexc, indicate the low basicity of these species in the excited state. Triggered by semiempirical theoretical calculations, the energetic splitting between the two lowest excited states could be related to the excited-state acidity and points to alterations in the electronic mixing of locally excited and charge-transfer states, caused by the substituents. Differences between the threefold negatively charged pyranine and the new neutral photoacids are also discussed.

 Please wait while we load your content...
Please wait while we load your content...