On the chemical state of Co oxide electrocatalysts during alkaline water splitting†
Abstract
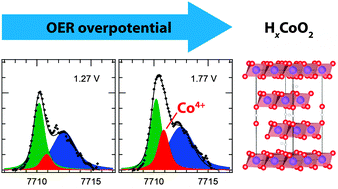
Resonant inelastic X-ray scattering and high-resolution X-ray absorption spectroscopy were used to identify the chemical state of a Co electrocatalyst in situ during the oxygen evolution reaction. After anodic electrodeposition onto Au(111) from a Co2+-containing electrolyte, the chemical environment of Co can be identified to be almost identical to CoOOH. With increasing potentials, a subtle increase of the Co oxidation state is observed, indicating a non-stoichiometric composition of the working OER catalyst containing a small fraction of Co4+ sites. In order to confirm this interpretation, we used density functional theory with a Hubbard-U correction approach to compute X-ray absorption spectra of model compounds, which agree well with the experimental spectra. In situ monitoring of catalyst local structure and bonding is essential in the development of structure–activity relationships that can guide the discovery of efficient and earth abundant water splitting catalysts.

 Please wait while we load your content...
Please wait while we load your content...