To the pore and through the pore: thermodynamics and kinetics of helium in exotic cubic carbon polymorphs†
Abstract
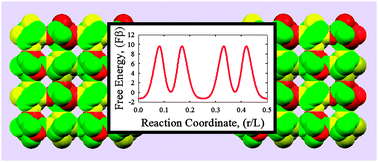
Applying pore size analysis, Monte Carlo simulations, and transition state theory, we study the molecular sieving properties of recently discovered crystalline exotic cubic carbon allotropes (Hu et al., J. Phys. Chem. C, 2012, 116, 24233–24238) at 298 K and infinite dilution. The fcc-C10 cubic carbon crystal shows unusual molecular sieving characteristics. The carbon cavities of the fcc-C10 cubic carbon polymorph (with an effective size of ∼3.5–4 Å) are kinetically closed to common gaseous contaminants of He fluid (including: Ne, Ar, H2, and CO). Because the sizes of nanowindows connecting carbon cavities are comparable with the effective size of a He atom (∼2.556 Å), we predict a significant resistance to self-diffusion of the He in the fcc-C10 crystal. Computed self-diffusion coefficients ∼1.3 × 10−6–1.3 × 10−7 cm2 s−1 for He inside fcc-C10 fall in the range characteristic of molecular diffusion in zeolites. Infrequent “jumps” of He atoms between neighboring carbon cavities and kinetic rejection of other gaseous particles indicate potential application of the fcc-C10 carbon polymorph for kinetic molecular sieving of He near ambient temperatures. The theoretical results presented here are useful for correct interpretation of the pore volumes of carbon molecular sieves measured from helium porosimetry.

 Please wait while we load your content...
Please wait while we load your content...