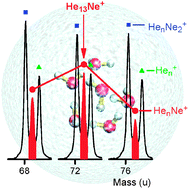
Helium nanodroplets are doped with neon and ionized by electrons. The size-dependence of the ion abundance of HenNex+, identified in high-resolution mass spectra, is deduced for complexes containing up to seven neon atoms and dozens of helium atoms. Particularly stable ions are inferred from anomalies in the abundance distributions. Two pronounced anomalies at n = 11 and 13 in the HenNe+ series confirm drift-tube data reported by Kojima et al. [T. M. Kojima et al., Z. Phys. D, 1992, 22, 645]. The discrepancy with previously published spectra of neon-doped helium droplets, which did not reveal any abundance anomalies [T. Ruchti et al., J. Chem. Phys., 1998, 109, 10679–10687; C. A. Brindle et al., J. Chem. Phys., 2005, 123, 064312], is most likely due to limited mass resolution, which precluded unambiguous analysis of contributions from different ions with identical nominal mass. However, calculated dissociation energies of HenNe+ reported so far do not correlate with the present data, possibly because of challenges in correctly treating the linear, asymmetric [He–Ne–He]+ ionic core in HenNe+. Anomalies identified in the distributions of HenNex+ for x > 1, including prominent ones at He12Ne2+ and He14Ne2+, may help to better understand solvation of Ne+ and Nex+ in helium.

 Please wait while we load your content...
Please wait while we load your content...