Enhancing the electrochemical kinetics of high voltage olivine LiMnPO4 by isovalent co-doping†
Abstract
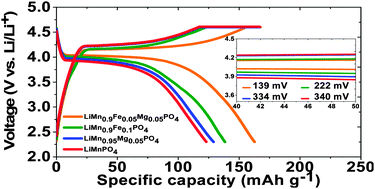
We report here doping of Fe2+ and/or Mg2+ in LiMnPO4 cathode material to enhance its lithium storage performance and appraise the effect of doping. For this purpose, LiMn0.9Fe(0.1−x)MgxPO4/C (x = 0 and 0.05) and LiMn0.95Mg0.05PO4/C have been prepared by a ball mill assisted soft template method. These materials were prepared with similar morphology, particle size and carbon content. Amongst them, the isovalent co-doped LiMn0.9Fe0.05Mg0.05PO4/C sample shows better electrochemical performance compared to LiMn0.9Fe0.1PO4/C and LiMn0.95Mg0.05PO4/C samples. For instance, a lithium storage capacity of 159 mA h g−1 is obtained at 0.1 C for LiMn0.9Fe0.05Mg0.05PO4/C material with a relatively low polarization of ∼139 mV. This is in sharp contrast to LiMn0.9Fe0.1PO4/C and LiMn0.95Mg0.05PO4/C which show only 136.8 and 128.4 mA h g−1 at 0.1 C with the polarization of ∼222 and 334 mV respectively. Further, the LiMn0.9Fe0.05Mg0.05PO4/C electrode delivers discharge capacities of 155.8, 141.4, 118.8, 104.6, 81.4 and 51.8 mA h g−1 at 0.2, 0.5, 1, 2, 5 and 10 C respectively. This electrode material also retains a capacity of 116 mA h g−1 at 1 C after 200 cycles, which is 96% of its initial capacity. Such improved cycling stability of LiMn0.9Fe0.05Mg0.05PO4/C is attributed to the suppressed Mn dissolution in the electrolyte compared to the other samples. Further, during the Li extraction process, delithiated phases created from the Fe2+/Fe3+ redox reaction (∼3.45 V) favor enhanced electrochemical activity of the succeeding Mn2+/Mn3+ redox couples. The fully charged state (4.6 V) contains a partially lithiated phase owing to the presence of electrochemically inactive Mg2+. The presence of such lithiated phase provides a favourable environment for the subsequent lithium insertion process. We also observe improved electronic conductivity and Li-ion diffusion for the co-doped sample compared to LiMnPO4 doped with either Fe2+ or Mg2+ by impedance measurements. The improved storage performance of co-doped LiMnPO4 is thus explained in terms of (i) favorable extraction and insertion reactions and (ii) enhanced transport properties.

 Please wait while we load your content...
Please wait while we load your content...