The oxygen migration in the apatite-type lanthanum silicate with the cation substitution†
Abstract
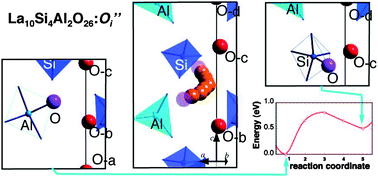
A theoretical model is proposed to determine the effects of Si substitution with Al on the oxygen diffusion in apatite-type lanthanum silicates based on density-functional theory (DFT) calculations for La10(SiO4)4(AlO4)2O2. Substitution changes the stable configuration for excess oxygen from the split interstitial to a new cluster form with the original cluster. Al doping completely changes the migration mechanism from the interstitialcy one, which was proposed for the La9.33(SiO4)6O2 starting material, to a mechanism which contains an interstitial process. Nevertheless, the migration barrier is calculated to be 0.81 eV, which indicates small changes in oxygen conduction and is consistent with the observations. The present study indicates that the cation substitution on silicon site alone does not promise the improvement of the oxide ion conduction in the lanthanum silicate.

 Please wait while we load your content...
Please wait while we load your content...