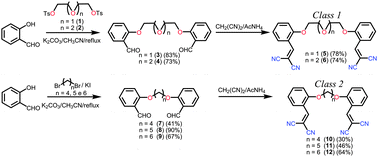
Two series of flexible dicyanomethylene compounds, specifically, class 1 and class 2 compounds, have been designed and synthesised. In class 1 compounds, the dicyanomethylene groups are separated by glycol chain spacers of different lengths, whereas, in class 2 compounds, the spacers are alkyl linkers of different lengths. The notion underlying the design of these compounds is that in class 1 molecules, the spacers contain donor oxygen atoms that could not only form hydrogen bonds during the course of crystal packing but also promote withdrawing effects that modify the photophysical and electrochemical properties of these molecules in solution; in contrast, these effects would be absent for class 2 molecules. However, this study revealed that, with respect to crystal packing, the size of the spacers and their even and odd numbers of atoms are more important than their chemical nature. All of the synthesised compounds exhibited blue emission in the solid state and in CH2Cl2 solutions. The photophysical and electrochemical properties of these compounds in solution were not significantly affected by the type and length of the spacer that was used in each molecule. In the solid state, however, the compound with the shortest spacer showed the highest Stokes shift. The electronic transitions for the synthesized compounds in solution were explained by density functional theory and time-dependent density functional theory calculations, which indicated that the methylene moieties control the properties of both classes of compounds and that the spacers do not conjugate with the end groups. These two series of flexible dicyanomethylene compounds could be utilised as molecular building blocks for the development of new solids with novel properties.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...