Freezing out all-optical poling dynamics of azophenylcarbazole molecules in polycarbonate†
Abstract
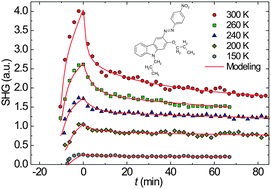
In this work we have extended the application of a theoretical model describing the processes of all-optical poling of isomerizable molecules while taking into consideration the thermoisomerization related findings presented in the literature. A model describing all-optical poling transients using a three relaxation rate approach was contrasted with the experimental results of azophenylcarbazole doped polycarbonate measured in a wide range of temperatures from 150 to 300 K, thus covering the β transition for the host. By means of a long timescale and low temperature, we were able to better resolve the thermoisomerization and orientational diffusion processes. The cis → trans thermoisomerization relaxation rates k1 and k2 were obtained in the range 10−5 to 10−2 s−1 and the relaxation rate k3 of the orientational diffusion of the trans isomer in the range 10−6 to 10−4 s−1. The rates exhibited diverse temperature dependent behaviors: the two lowest (k2,k3) manifested Arrhenius type dependencies (Ek2 = 157 meV), whereas, the highest (k1) showed a temperature dependence that is non-Arrhenius, or undistinguished for our experimental conditions. The latter was interpreted using the geometrical “adjustment” model. By investigating

 Please wait while we load your content...
Please wait while we load your content...