The effect of oxygen vacancies on water wettability of a ZnO surface
Abstract
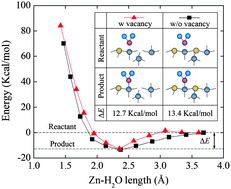
In this study, the effect of oxygen vacancies on the water wettability of a hydrated ZnO(100) surface has been examined via molecular dynamics simulations with a reactive force field (ReaxFF). The results show that the oxygen vacancies on the ZnO surface change the structures of the ZnO surface and subsequently its water

 Please wait while we load your content...
Please wait while we load your content...