Estimation of electrode ionomer oxygen permeability and ionomer-phase oxygen transport resistance in polymer electrolyte fuel cells
Abstract
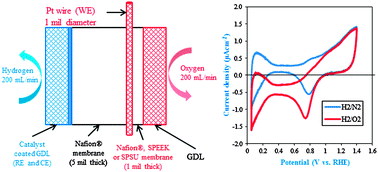
The oxygen permeability of perfluorinated and hydrocarbon polymer electrolyte membranes (PEMs; Nafion®, SPEEK and SPSU), which are used as electrolytes and electrode ionomers in polymer electrolyte fuel cells (PEFCs), was estimated using chronoamperometry using a modified fuel cell set-up. A thin, cylindrical microelectrode was embedded into the PEM and used as the working electrode. The PEM was sandwiched between 2 gas diffusion electrodes, one of which was catalyzed and served as the counter and pseudo-reference electrode. Independently, from fuel cell experiments, the oxygen transport resistance arising due to transport through the ionomer film covering the catalyst active sites was estimated at the limiting current and decoupled from the overall mass transport resistance. The in situ oxygen permeability measured at 80 °C and 75% RH of perfluorinated ionomers such as Nafion® (3.85 × 1012 mol cm−1 s−1) was observed to be an order of magnitude higher than that of hydrocarbon-based PEMs such as SPEEK (0.27 × 1012 mol cm−1 s−1) and SPSU (0.15 × 1012 mol cm−1 s−1). The obtained oxygen transport (through ionomer film) resistance values (Nafion® – 1.6 s cm−1, SPEEK – 2.2 s cm−1 and SPSU – 3.0 s cm−1; at 80 °C and 75% RH) correlated well with the measured oxygen permeabilities in these ion-containing polymers.

 Please wait while we load your content...
Please wait while we load your content...