Vibrational Stark effect spectroscopy reveals complementary electrostatic fields created by protein–protein binding at the interface of Ras and Ral
Abstract
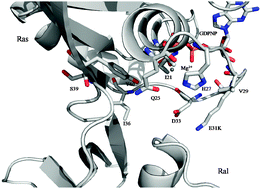
Electrostatic fields at the interface of the GTPase H-Ras (Ras) docked with the Ras binding domain of the

 Please wait while we load your content...
Please wait while we load your content...