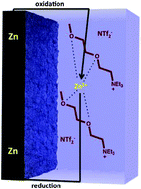
Advanced, high energy-density, metal–air rechargeable batteries, such as zinc–air, are of intense international interest due to their important role in energy storage applications such as electric and hybrid vehicles, and to their ability to deal with the intermittency of renewable energy sources such as solar and wind. Ionic liquids offer a number of ideal thermal and physical properties as potential electrolytes in such large-scale energy storage applications. We describe here the synthesis and characterisation of a family of novel “chelating” ILs designed to chelate and solubilize the zinc ions to create electrolytes for this type of battery. These are based on quaternary alkoxy alkyl ammonium cations of varying oligo-ether side chains and anions such as p-toluene sulfonate, bis(trifluoromethylsulfonyl)amide and dicyanoamides. This work shows that increasing the ether chain length in the cation from two to four oxygens can increase the ionic conductivity and reduce the melting point from 67 °C to 15 °C for the tosylate system. Changing the anion also plays a significant role in the nature of the zinc deposition electrochemistry. We show that zinc can be reversibly deposited from [N222(20201)][NTf2] and [N222(202020201)][NTf2] beginning at −1.4 V and −1.7 V vs. SHE, respectively, but not in the case of tosylate based ILs. This indicates that the [NTf2] is a weaker coordinating anion with the zinc cation, compared to the tosylate anion, allowing the coordination of the ether chain to dominate the behavior of the deposition and stripping of zinc ions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...