A mechanistic study supports a two-step mechanism for peptide bond formation on the ribosome†
Abstract
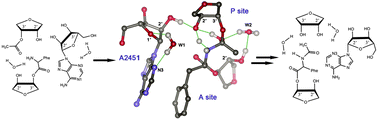
We report the feasible pathways of the quaternary model system for the ribosome-catalyzed PT reaction obtained by density functional calculations. Our results indicate that the step from the reactant complex to the first six-membered TS involving a

 Please wait while we load your content...
Please wait while we load your content...