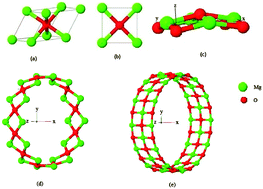
The structural, vibrational and response properties of the (n,0) and (m,m) MgO nanotubes are computed by using a Gaussian type basis set, a hybrid functional (B3LYP) and the CRYSTAL09 code. Tubes in the range 6 ≤ n ≤ 140 and 3 ≤ m ≤ 70 were considered, being n = 2 × m the number of MgO units in the unit cell (so, the maximum number of atoms is 280). Tubes are built by rolling up the fully relaxed 2-D conventional cell (2 MgO units, with oxygen atoms protruding from the Mg plane alternately up and down by 0.38 Å). The relative stability of the (n,0) with respect to the (m,m) family, the relaxation energy and equilibrium geometry, the band gap, the IR vibrational frequencies and intensities, and the electronic and ionic contributions to the polarizability are reported. All these properties are shown to converge smoothly to the monolayer values. Absence of negative vibrational frequencies confirms that the tubes have a stable structure. The parallel component of the polarizability α∥ converges very rapidly to the monolayer value, whereas α⊥ is still changing at n = 140; however, when extrapolated to very large n values, it coincides with the monolayer value to within 1%. The electronic contribution to α is in all cases (α∥ and α⊥; 6 ≤ n ≤ 140) smaller than the vibrational contribution by about a factor of three, at variance with respect to more covalent tubes such as the BN ones, for which the ratio between the two contributions is reversed.

 Please wait while we load your content...
Please wait while we load your content...