The formation of peroxide degradation products of photochromic triphenylimidazolyl radical-dimers†
Abstract
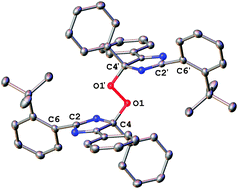
Following the recent report of Abe and co-workers (Phys. Chem. Chem. Phys., 2012, 14, 5855) of the isolation of a bridging peroxide of a naphthalene-tethered bisimidazolyl diradical, it is reported herein that this degradation pathway is a more general phenomenon for the chromic dimers of 2,4,5-triphenylimidazolyl radical (TPIR) materials, with non-tethered TPIRs forming similar oxygen adducts. The peroxides of two derivatives have been characterised by single crystal X-ray diffraction (SC-XRD) and it is identified that the 4-position of the imidazolyl ring is the site susceptible to reaction with oxygen. Furthermore, mass spectrometry has been used to show that for a range of five known, non-tethered derivatives, peroxide formation can be detected within 30 minutes when samples are irradiated under an oxygen atmosphere, thus presenting a significant challenge to the long term use of this class of material in colour-switching device applications.

 Please wait while we load your content...
Please wait while we load your content...