Screening of transition and post-transition metals to incorporate into copper oxide and copper bismuth oxide for photoelectrochemical hydrogen evolution†
Abstract
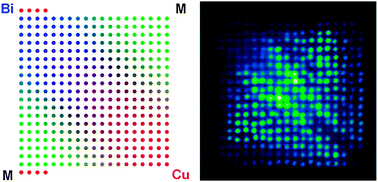
A new dispenser and scanner system is used to create and screen Bi–M–Cu oxide arrays for cathodic photoactivity, where M represents 1 of 22 different transition and post-transition metals. Over 3000 unique Bi : M : Cu atomic ratios are screened. Of the 22 metals tested, 10 show a M–Cu oxide with higher photoactivity than CuO and 10 show a Bi–M–Cu oxide with higher photoactivity than CuBi2O4. Cd, Zn, Sn, and Co produce the most photoactive M–Cu oxides, all showing a 200–300% improvement in photocurrent over CuO. Ag, Cd, and Zn produce the highest photoactivity Bi–M–Cu oxides with a 200–400% improvement over CuBi2O4. Most notable is a Bi–Ag–Cu oxide (Bi : Ag : Cu atomic ratio of 22 : 3 : 11) which shows 4 times higher photocurrent than CuBi2O4. This material is capable of evolving hydrogen under illumination in neutral electrolyte solutions at 0.6 V vs. RHE when Pt is added to the surface as an electrocatalyst.

 Please wait while we load your content...
Please wait while we load your content...