Hydrogen and halogen bonds are ruled by the same mechanisms†
Abstract
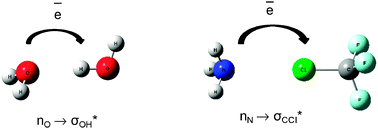
Hydrogen and halogen bonds are compared on the basis of ab initio calculations performed for complexes linked through these interactions. The Quantum Theory of Atoms in Molecules (QTAIM) and the Natural Bond Orbitals (NBO) method are applied for a deeper understanding of the nature of interactions. Both interactions are ruled by the same effects of hyperconjugation and rehybridization. In general for both kinds of interactions the same processes of the electron charge redistribution being the result of complexation are observed. As a consequence similar characteristics are also observed for the hydrogen and halogen bonds for example the increase of the positive charge of the atom being in contact with the Lewis base (hydrogen and chlorine or bromine for complexes analyzed here) and the decrease of its volume as a result of the complex formation. The halogen bond is enhanced by the charge assistance, similarly to the hydrogen bond.

 Please wait while we load your content...
Please wait while we load your content...