Destabilization of Mg–H bonding through nano-interfacial confinement by unsaturated carbon for hydrogen desorption from MgH2†
Abstract
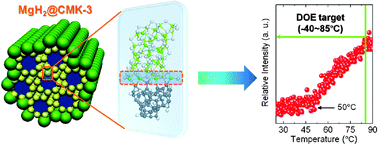
We propose a new mechanism for destabilizing Mg–H bonding by means of a combination of the size effect and MgH2–carbon scaffold interfacial bonding, and experimentally realize low temperature hydrogen release starting from 50 °C using an MgH2@CMK-3 nanoconfinement system (37.5 wt% MgH2 loading amount). Based on computational calculations, it is found that the charge transfer from MgH2 to the carbon scaffold plays a critical role in the significant reduction of thermodynamics of MgH2

 Please wait while we load your content...
Please wait while we load your content...