Theoretical prediction of new noble-gas molecules FNgBNR (Ng = Ar, Kr, and Xe; R = H, CH3, CCH, CHCH2, F, and OH)†
Abstract
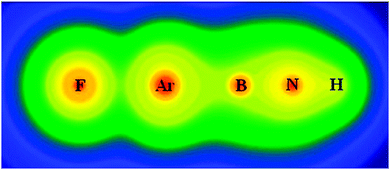
We have computationally predicted a new class of stable noble-gas molecules FNgBNR (Ng = Ar, Kr, Xe; R = H, CH3, CCH, CHCH2, F, and OH). The FNgBNR were found to have compact structures with F–Ng bond lengths of 1.9–2.2 Å and Ng–B bond lengths of ∼1.8 Å. The endoergic three-body dissociation energies of FNgBNH to F + Ng + BNH were calculated to be 12.8, 31.7, and 63.9 kcal mol−1, for Ng = Ar, Kr, and Xe, respectively at the CCSD(T)/CBS level. The energy barriers of the exoergic two-body dissociation to Ng + FBNH were calculated to be 16.1, 24.0, and 33.2 kcal mol−1 for Ng = Ar, Kr, and Xe, respectively. Our results showed that the dissociation energetics is relatively insensitive to the identities of the terminal R groups. The current study suggested that a wide variety of noble-gas containing molecules with different types of R groups can be thermally stable at low temperature, and the number of potentially stable noble-gas containing molecules would thus increase very significantly. It is expected some of the FNgBNR molecules could be identified in future experiments under cryogenic conditions in noble-gas matrices or in the gas phase.

 Please wait while we load your content...
Please wait while we load your content...