Hydrogen storage by physisorption on dodecahydro-closo-dodecaboranes
Abstract
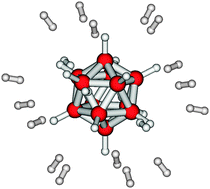
Hydrogen physisorption on dodecahydro-closo-dodecaborane units is studied using ab initio quantum chemical calculations based on Møller–Plesset perturbation theory. After adding zero-point energy corrections, the adsorption energy due to the charge–quadrupole and the charge-induced dipole interaction is somewhat larger than the more common dispersion interaction with spacer molecules in molecular framework compounds. Furthermore, the energy landscape on the surface of the near-spherical B12H122− permits considerable residual dynamics with corresponding configurational entropy that releases partly the requirements on the magnitude of the adsorption energy. If it can be made fully accessible in an open architecture the system promises an enormous storage capacity. An experimental test for Cs2B12H12 dispersed in the cages of a dealuminated faujasite

 Please wait while we load your content...
Please wait while we load your content...