Theoretical design and experimental implementation of Ag/Au electrodes for the electrochemical reduction of nitrate†
Abstract
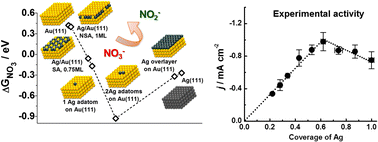
The current imbalance in the biogeochemical cycle of nitrogen is as serious as that of carbon. One way to mitigate this problem is through the electrochemical reduction of nitrates under mild conditions, which is an appealing though not fully understood process. Therefore, deeper insight into the electrocatalytic reaction mechanism is needed to optimize this process. Here we thoroughly analyse the adsorption energy of nitrate with DFT calculations on various surface facets of pure Au, Ag, and their near-surface and surface alloys, as the adsorption and subsequent reduction of nitrate are thought to be rate limiting in the electrocatalytic reaction. The observed systematic trends allow prediction of the surface with highest electrocatalytic activity for the reduction of nitrate. This prediction was verified experimentally by depositing sub-monolayer amounts of Ag on polycrystalline Au electrodes. We observe a well-defined volcano curve which correlates the amount of Ag deposited on the surface with the current density at a fixed potential, with the peak activity around 2/3 ML Ag surface coverage. The electrocatalytic activity and stability of the bimetallic Ag–Au systems, found through the interplay of theoretical modelling and empirical observations, serve as a clear example for the rational design of novel catalytic materials and confirm the key role that the adsorption of nitrate plays in the overall nitrate reduction rate.
- This article is part of the themed collection: PCCP Emerging Investigator Lectureship Award Winners

 Please wait while we load your content...
Please wait while we load your content...