Prediction of (TiO2)x(Cu2O)y alloys for efficient photoelectrochemical water splitting†
Abstract
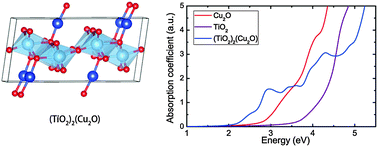
The formation of (TiO2)x(Cu2O)y solid-solutions is investigated using a global optimization evolutionary algorithm. First-principles calculations based on density functional theory are then used to gain insight into the electronic properties of these alloys. We find that: (i) Ti and Cu in (TiO2)x(Cu2O)y alloys have similar local environments as in bulk TiO2 and Cu2O except for (TiO2)(Cu2O) which has some trigonal-planar Cu ions. (ii) The predicted optical band gaps are around 2.1 eV (590 nm), thus having much better performance in the absorption of visible light compared with both binary

 Please wait while we load your content...
Please wait while we load your content...