Alkylation of complementary ribonucleotides in nanoreactors†
Abstract
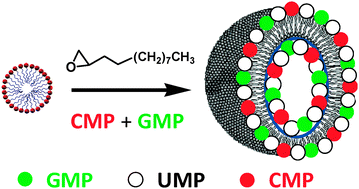
The aim of the present study was to provide experimental evidence that base pairing, commonly occurring between nucleic bases in more complex supramolecular arrangements, may affect the reaction pathways associated with the alkylation of bases themselves. In pursuit of this aim, dilute aqueous solutions of Cytidine- (CMP) and Guanosine-Mono-Phosphate (GMP) as single reactants or in an equimolar mixture were treated with the electrophilic alkylating agent 1,2-Dodecyl-Epoxide (DE), which was preventively dispersed into micellar solutions prepared with the cationic surfactant hexadecyltrimethylammonium bromide (CTAB). In the early stage of the reaction, CTAB micelles acted as micro-heterogeneous nanoreactors, but as the reaction progressed the systems evolved toward the formation of polydisperse aggregates, whose size and surface-charge properties were monitored as a function of reaction time. From mass spectrometry analyses, it was found that the deamination of cytosine, a side reaction related to the alkylation of the amino group of CMP, was reduced when both the complementary ribonucleotides were present in the same reaction mixture. The involvement of specific sites able to establish C:G interactions (possibly via H-bonding or π–π stacking) could explain the reduced reactivity occurring at the level of some of the nucleophilic centers responsible for molecular recognition.

 Please wait while we load your content...
Please wait while we load your content...