Assessment of permethylated transition-metal sandwich complexes as internal reference redox systems in ionic liquids†
Abstract
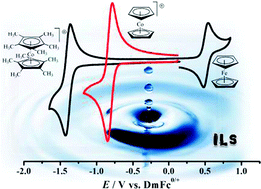
This work reports the voltammetric behaviour of decamethylcobaltocenium, DmCc+, in different ionic liquids for the first time. Its redox potential was studied relative to that of decamethylferrocene, DmFc, and it is shown that the difference in the mid-point potential between these two permethylated transition-metal sandwich complexes is independent of the ionic liquid composition. A variable difference in mid-point potential, in contrast, was observed for ferrocene and cobaltocenium relative to that of DmFc in similar ionic liquids. In addition, different limitations in the application of DmFc0/+ and DmCc+/0 couples as internal reference redox systems in ILs are discussed. From these, the observed spontaneous reaction between DmFc and oxygen leads to important implications toward the establishment of particular conditions for DmFc applications.
- This article is part of the themed collection: Electrochem 2012 "Electrochemical Horizons"

 Please wait while we load your content...
Please wait while we load your content...