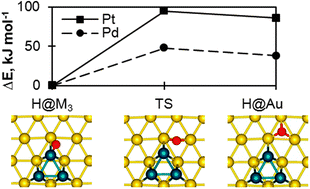
Motivated by the use of electrodes modified at the nanoscale by supported metal species, we studied computationally how the reactivity changes in such a composite system compared to the reactivity of the individual systems, metal clusters and metal surfaces. Specifically, we examined hydrogen adsorption on and hydrogen spillover from Au- and Cu-supported Pt3 and Pd3 clusters, using a method based on Density Functional Theory. Two distinctive types of sites were found for the adsorption of atomic hydrogen: (i) on the supported clusters and (ii) at the cluster–substrate interfaces. The adsorption energy of hydrogen on the supported clusters is ∼20 kJ mol−1 smaller when the cluster is supported by Cu instead of Au. In contrast, the substrate has no effect on hydrogen adsorbed at the cluster–substrate interfaces. Adsorbed Pt3 and Pd3 clusters locally modify the reactivity of the substrates as quantified by the reduced adsorption energy of hydrogen compared to the corresponding clean substrate. Hydrogen dissociative adsorption followed by spillover is thermodynamically and kinetically favored for clusters supported on a Cu surface, but not on Au. Moreover, spillover of hydrogen is more likely from metal-supported Pd than Pt clusters as revealed by barriers that are calculated 40–50 kJ mol−1 lower in energy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...