Geometry determination of complexes in a molecular liquid mixture using electron–vibration–vibration two-dimensional infrared spectroscopy with a vibrational transition density cube method
Abstract
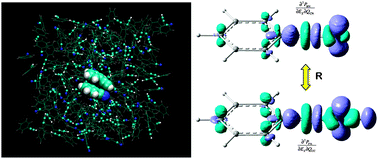
We demonstrate the use of a new vibrational transition density cube (VTDC) method for determining the geometry of complexes in a molecular liquid mixture from electron–vibration–vibration two-dimensional infrared (EVV

 Please wait while we load your content...
Please wait while we load your content...